-
Home
-
About JCTR
-
Gold Open Access
-
Issues
-
Editorial board
-
Author guidelines
-
Publication fees
-
Online first
-
Special issues
-
News
-
Publication ethics
-
Partners
-
Submit your manuscript
-
Submit your review report
-
Editorial Office
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. ISSN print: 2382-6533 ISSN online: 2424-810X
Volume 7 Issue 1
Biomarkers of mitotoxicity after acute liver injury: Further insights into the interpretation of glutamate dehydrogenase
Mitchell R. McGill*, and Hartmut Jaeschke
McGill et al. J Clin Transl Res 2021; 7(1):5
Published online: January 27, 2021
Abstract
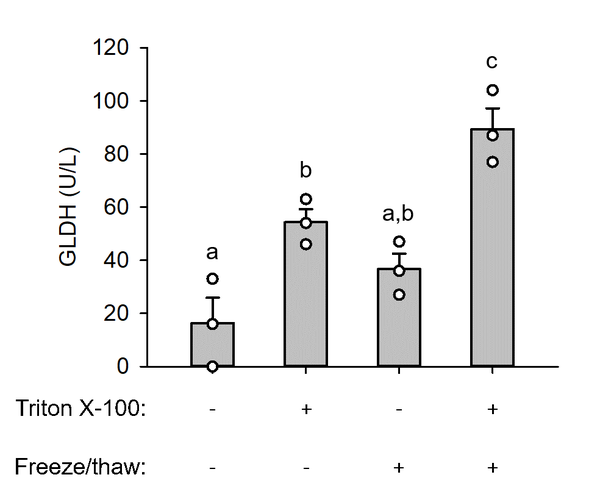
Background: Acetaminophen (APAP) is a popular analgesic, but overdose causes acute liver injury and sometimes death. Decades of research have revealed that mitochondrial damage is central in the mechanisms of toxicity in rodents, but we know much less about the role of mitochondria in humans. Due to the challenge of procuring liver tissue from APAP overdose patients, non-invasive mechanistic biomarkers are necessary to translate the mechanisms of APAP hepatotoxicity from rodents to patients. It was recently proposed that the mitochondrial matrix enzyme glutamate dehydrogenase (GLDH) can be measured in circulation as a biomarker of mitochondrial damage. Early observations revealed that damaged mitochondria release their contents into the cytosol. It follows that those mitochondrial molecules become freely detectable in blood after cell death. On the other hand, intact mitochondria would not release their matrix contents and can be removed from serum or plasma by high-speed centrifugation. However, a recent study cast doubt on the interpretation of GLDH as a mitotoxicity biomarker by demonstrating that neither high-speed centrifugation nor repeated freezing and thawing to lyse mitochondria alter GLDH activity in serum from mice with drug-induced liver injury.
Aim: Here, we briefly review the evidence for mitochondrial damage in APAP hepatotoxicity and demonstrate that removal of intact mitochondria by centrifugation does not alter measured GLDH activity simply because GLDH within the mitochondrial matrix is not accessible for measurement. In addition, we show that freezing and thawing is insufficient for complete lysis of mitochondria.
Relevance for patients: Our literature review and data support the interpretation that circulating GLDH is a biomarker of mitochondrial damage. Such mechanistic biomarkers are important to translate preclinical research to patients.
DOI: http://dx.doi.org/10.18053/jctres.07.202101.005
Author affiliation
1. Department of Environmental Health Sciences, Fay W. Boozman College of Public Health, University of Arkansas for Medical Sciences, 4301 W. Markham St, Little Rock, AR, USA, 72205
2. Department of Pharmacology and Toxicology, College of Medicine, University of Arkansas for Medical Sciences, 4301 W. Markham St., Little Rock, AR, USA, 72205
3. Department of Pharmacology, Toxicology and Therapeutics, University of Kansas Medical Center, 3901 Rainbow Blvd., Kansas City, KS, USA, 66160
*Corresponding author
Mitchell R. McGill
Department of Environmental Health Sciences & Department of Pharmacology and Toxicology, University of Arkansas for Medical Sciences, Little Rock, AR, USA
Tel: +1 501-526-6696
Email: mmcgill@uams.edu
Handling editor:
Michal Heger
Department of Pharmaceutics, Utrecht University, the Netherlands
Department of Pharmaceutics, Jiaxing University Medical College, Zhejiang, China

