-
Home
-
About JCTR
-
Gold Open Access
-
Issues
-
Editorial board
-
Author guidelines
-
Publication fees
-
Online first
-
Special issues
-
News
-
Publication ethics
-
Partners
-
Submit your manuscript
-
Submit your review report
-
Editorial Office
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. ISSN print: 2382-6533 ISSN online: 2424-810X
Volume 7 Issue 6
High affinity monoclonal antibody targeting Siglec-15 for cancer immunotherapy
Fei He, Na Wang, Jiangwei Li, Luanying He, Zhao Yang, Jiandong Lu, Guoliang Xiong, Changyuan Yu*, Shihui Wang*
He et al. J Clin Transl Res 2021; 7(6):10
Published online: November 16, 2021
Abstract
Background & aim: Recently, Siglec-15 has been proved as a novel immune suppressor and a potential target for normalization cancer immunotherapy, which is non-redundant to the well-known PD-L1/PD-1 pathway. Herein, anti-Siglec-15 mAb, a monoclonal antibody (mAb) with high affinity against Siglec-15, was prepared.
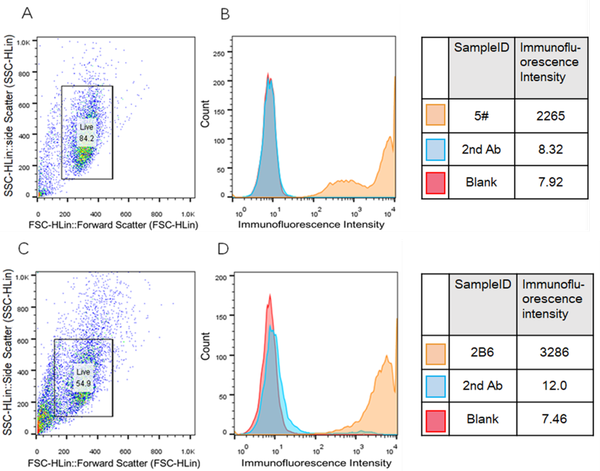
Methods: The engineered CHO-K1 Siglec-15 cell line was constructed to heterologously expressed Siglec-15 for the affinity test with the mAb. Antigens Siglec-15-mIgG and Siglec-15-his were recombinantly expressed by 293F cells and purified by HPLC. Hybridoma cell line against Siglec-15 was prepared and validated by ELISA and fluorescent-activated cell sorting (FACS). Finally, the anti-Siglec-15 mAb was produced, purified, and confirmed by SDS-PAGE, ELISA, and FACS.
Results: The EC50 of the anti-Siglec-15 mAb with Siglec-15 is 76.65 ng/mL, lower than that of the positive control 5G12 (90.7 ng/mL), indicating a high affinity of the anti-Siglec-15 mAb. In vitro and in vivo studies verified that the anti-Siglec-15 mAb blocks the Siglec-15-mediated suppression of T cell and moderately prevents the tumor growth.
Conclusions: The anti-Siglec-15 mAb can be considered as an effective immunotherapy for tumor suppression.
Relevance for patients: The anti-Siglec-15 mAb prepared in this study is useful as an immune checkpoint inhibitor against Siglec-15 for normalization cancer immunotherapy. This immunotherapy provides an alternative treatment for cancer patients who are refractory to the well-known PD-L1/PD-1-targeting therapies.
DOI: http://dx.doi.org/10.18053/jctres.07.202106.010
Author affiliation
1. College of Life Science and Technology, Beijing University of Chemical Technology, Beijing 100029, China
2. College of Life Science, Key Laboratory of Protection and Utilization of Biological Resources in Tarim Basin of Xinjiang Production and Construction Corps, Tarim University, Alar 843300, Xinjiang, China
3. Shenzhen Traditional Chinese Medicine Hospital, Guangzhou University of Chinese Medicine, Shenzhen, 518033, Guangdong, China
*Corresponding author
Changyuan Yu
Shihui Wang
College of Life Science and Technology, Beijing University of Chemical Technology, Beijing 100029, China
Tel: +86-10-64421335
Fax: +86-10-64421335
Email: wangshihui@mail.buct.edu.cn; yucy@mail.buct.edu.cn
Handling editor:
Michal Heger
Department of Pharmaceutics, Utrecht University, the Netherlands
Department of Pharmaceutics, Jiaxing University Medical College, Zhejiang, China

