-
Home
-
About JCTR
-
Gold Open Access
-
Issues
-
Editorial board
-
Author guidelines
-
Publication fees
-
Online first
-
Special issues
-
News
-
Publication ethics
-
Partners
-
Submit your manuscript
-
Submit your review report
-
Editorial Office
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. ISSN print: 2382-6533 ISSN online: 2424-810X
Volume 8 Issue 2
Impact of AmaTea® Max on physiological measures and gaming performance in active gamers: a placebo-controlled, double-blind, randomized study
Richard J. Bloomer*, Keith R. Martin, Jacquelyn C. Pence
Bloomer et al. J Clin Transl Res; 8(2):5
Published online: March 1, 2022
Abstract
Background and aim: The activity of “gaming” has increased greatly in popularity in recent years, with many gamers using nutritional supplements to aid mood and gaming performance. We evaluated the impact of AmaTea® Max (referred to as AmaTea® throughout; a patented dietary supplement consisting of a blend of caffeine and polyphenol antioxidants), compared to both caffeine and a placebo, on gaming and cognitive performance in active gamers.
Methods: Subjects reported to the lab on three occasions, separated by approximately one week. On each day, they had baseline measurements taken and then played the game Fortnite for four one-hour periods. Measures of cognitive performance, gaming performance, heart rate and blood pressure, and blood cortisol were measured before and at selected times following gameplay.
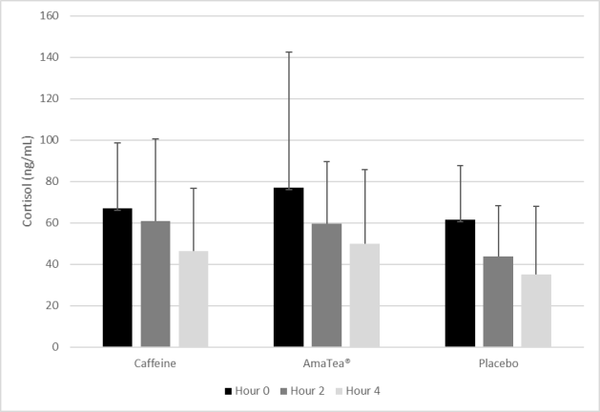
Results: Neither caffeine nor AmaTea® impacted gaming or cognitive performance in a statistically significant manner. However, a trend (p=0.075) was noted for the condition effect for kills/match, with values 21% higher for AmaTea® (1.84) compared to placebo (1.51), and 12% higher for AmaTea® compared to caffeine (1.63). Subjective mood was relatively unaffected, although a condition effect was noted for jittery (p=0.05), with values lower for placebo than for caffeine (p=0.02). Blood pressure was minimally elevated with both AmaTea® and caffeine, while cortisol followed the normal diurnal variation and was lower for placebo than AmaTea® and caffeine.
Conclusion: AmaTea® modestly increased kills/match during gameplay. It is possible that a different gaming stimulus, varied time of gameplay, or different dosage of the supplement may have yielded different results.
Relevance for Subjects: Active gamers who seek to use a dietary supplement for purposes of gaming performance may benefit slightly from ingestion of AmaTea® prior to gameplay, while experiencing greater vigor and lower fatigue as compared to placebo.
DOI: http://dx.doi.org/10.18053/jctres.08.202202.005
Author affiliation
Center for Nutraceutical and Dietary Supplement Research, College of Health Sciences, University of Memphis, Memphis, TN, USA
*Corresponding author
Richard J. Bloomer
106 Elma Roane Fieldhouse, University of Memphis, Memphis, TN 38152
Tel: 901-678-5638
Email: rbloomer@memphis.edu
Handling editor:
Michal Heger
Department of Pharmaceutics, Utrecht University, the Netherlands
Department of Pharmaceutics, Jiaxing University Medical College, Zhejiang, China

