-
Home
-
About JCTR
-
Gold Open Access
-
Issues
-
Editorial board
-
Author guidelines
-
Publication fees
-
Online first
-
Special issues
-
News
-
Publication ethics
-
Partners
-
Submit your manuscript
-
Submit your review report
-
Editorial Office
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. ISSN print: 2382-6533 ISSN online: 2424-810X
Volume 9 Issue 1
Bacopa monnieri supplementation has no effect on serum brain-derived neurotrophic factor (BDNF) levels but beneficially modulates NF-kB and CREB levels in healthy elderly subjects
Andrew P. Keegan*, Con Stough, Daniel Paris, Cheryl A. Luis, Laila Abdullah, Ghania Ait-ghezala, Fiona Crawford, Michael Mullan
Keegan et al. J Clin Transl Res 2023; 9(1):7
Published online: January 17, 2023
Abstract
Background and aim: Bacopa monnieri is an Ayurvedic herb that has been used for multiple conditions, most notably to augment cognition, particularly memory and attention. Multiple mechanisms, including raising brain-derived neurotrophic factor (BDNF), have been proposed and investigated in animal models that require translational studies in humans.
Methods: Bacopa was administered in an open-labeled study to cognitively healthy controls over a three-month period. Cognition and mood were assessed using the Montreal Cognitive Assessment (MoCA) and Geriatric Depression Scale (GDS) at the baseline and 3-month visit. Labs were assessed for safety and serum levels of mature (mBDNF) and proBDNF were quantified. In a subset of subjects, intracellular signaling processes were assessed using Western blot analysis.
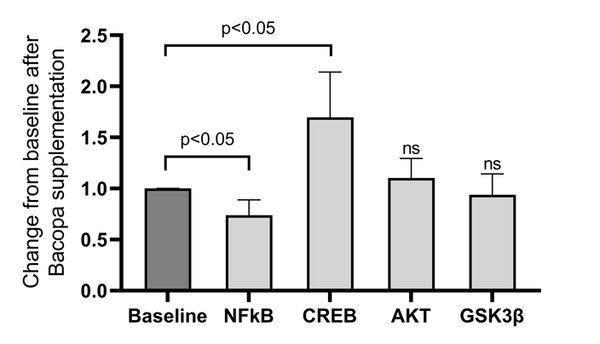
Results: Bacopa was provided to 35 subjects and was well-tolerated except for 4 (11%) subjects who early terminated due to known, reversible, gastrointestinal side effects (i.e. nausea, diarrhea). Over the 3 months, the GDS and the total MoCA did not significantly change, however, the delayed-recall subscale significantly improved (baseline: 3.8 ± 1.2, 3-months: 4.3 ± 0.9; p = 0.032). Serum mBDNF and proBDNF levels did not significantly change. Cyclic AMP response element-binding protein (CREB) phosphorylation significantly increased (p = 0.028) and p65 nuclear factor kappa B (NF-κB) phosphorylation significantly decreased (p = 0.030).
Conclusion: These results suggest Bacopa may exert an anti-inflammatory effect via NF-κB and improve intracellular signaling processes associated with synaptogenesis (CREB). Future placebo-controlled studies are recommended.
Relevance for patients: Bacopa monnieri will require larger, blinded trials to better understand potential mechanisms, interactions, and utilization.
DOI: http://dx.doi.org/10.18053/jctres.09.202301.007
Author affiliation
1. The Roskamp Institute, Sarasota, Florida, United States of America
2. Swinburne Centre for Human Psychopharmacology, Swinburne University, Melbourne, Australia
*Corresponding author
Andrew P. Keegan
The Roskamp Institute, 2040 Whitfield Avenue, Sarasota, Florida 34243, United States of America.
Tel: +1 941-752-2949
Fax: +1 941-752-2948
E-mail: akeegan@roskampinstitute.or
Handling editor:
Michal Heger
Department of Pharmaceutics, Utrecht University, the Netherlands
Department of Chemistry, Utrecht University, Utrecht, the Netherlands
Department of Pathology, Erasmus Medical Center, the Netherlands
Department of Pharmaceutics, Jiaxing University Medical College, Zhejiang, China

