-
Home
-
About JCTR
-
Gold Open Access
-
Issues
-
Editorial board
-
Author guidelines
-
Publication fees
-
Online first
-
Special issues
-
News
-
Publication ethics
-
Partners
-
Submit your manuscript
-
Submit your review report
-
Editorial Office
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. ISSN print: 2382-6533 ISSN online: 2424-810X
Volume 9 Issue 4
Effect of a 20% intravenous fat emulsion therapy on pregnancy outcomes in women with RPL or RIF undergoing IVF/ICSI: a systematic review and meta-analysis
Greg Marchand*, Ahmed Taher Masoud, Hollie Ulibarri, Amanda Arroyo, Catherine Coriell, Sydnee Goetz, Carmen Moir, Atley Moberly, Daniela Gonzalez, Madison Blanco, Harry Randall Craig
Marchand et al. J Clin Transl Res 2023; 9(4):23-00060
Published online: July 12, 2023
Abstract
Background and aim: To evaluate the efficacy a 20% intravenous fat emulsion therapy in women suffering from recurrent pregnancy loss or recurrent implantation failure (RPL/RIF) who are undergoing in vitro fertilization (IVF) or intracytoplasmic sperm injection (ICSI).
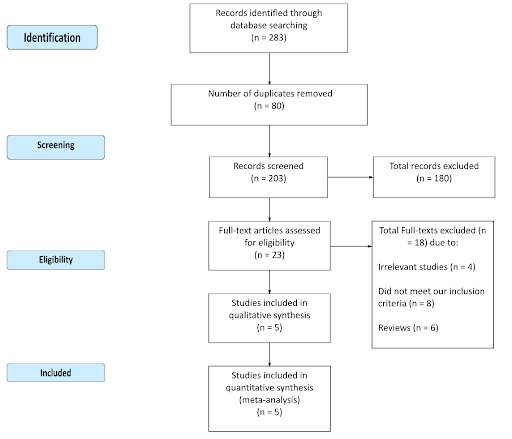
Methods: We searched Cochrane Library, ISI Web of Science, MEDLINE, ClinicalTrials.gov, PubMed, and Scopus using relevant keywords during February 2020 for randomized controlled trials (RCTs) comparing the therapy versus placebo or no intervention in women suffering from RPL/RIF and undergoing IVF/ICSI.
Results: We included five RCTs with 840 patients. The intravenous fat emulsion therapy was significantly effective in increasing clinical pregnancy rates compared to the control group (RR=1.48, 95% CI [1.23,1.79], p<0.001). Furthermore, ongoing pregnancy and live birth rates were significantly higher with 20% intravenous fat emulsion therapy (RR=1.71, 95% CI [1.27,2.32], p=0.005) and (RR= 1.85, 95% CI [1.44, 2.38], p<0.001). Despite the statistically significant differences, the quality of evidence was only considered moderate, and this was primarily due to high risk of bias in the included RCTs.
Conclusion: Our review provides a moderate level of evidence that intravenous fat emulsion therapy is effective in improving reproductive outcomes among women with RPL/RIF performing IVF/ICSI techniques. Further investigation is required to ascertain optimal dosage and timing of administration.
Relevance for Patients: Women suffering from RPL or RIF may wish to consider discussing with their reproductive endocrinologist the addition of a 20% fat emulsion therapy to planned IVF or ICSI cycles, which may improve outcomes.
DOI: http://dx.doi.org/10.18053/jctres.09.202304.23-00060
Author affiliation
1. Marchand Institute for Minimally Invasive Surgery, Mesa, Arizona, United States of America
2. Fayoum University Faculty of Medicine, Fayoum, Egypt
3. Fertility Treatment Center, Tempe, Arizona, United States of America
*Corresponding author
Greg J. Marchand
Marchand Institute for Minimally Invasive Surgery, 10238 E. Hampton, Ste. 212, Mesa, AZ 85209, United States of America.
Tel: +1 4809990905; +1 4809990801
Email: gm@marchandinstitute.org
Handling editor:
Michal Heger
Department of Pharmaceutics, Utrecht University, the Netherlands
Department of Chemistry, Utrecht University, Utrecht, the Netherlands
Department of Pathology, Erasmus Medical Center, the Netherlands
Department of Pharmaceutics, Jiaxing University Medical College, Zhejiang, China

