-
Home
-
About JCTR
-
Gold Open Access
-
Issues
-
Editorial board
-
Author guidelines
-
Publication fees
-
Online first
-
Special issues
-
News
-
Publication ethics
-
Partners
-
Submit your manuscript
-
Submit your review report
-
Editorial Office
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. ISSN print: 2382-6533 ISSN online: 2424-810X
Volume 3 Special issue on negative results
Administration of DDAVP did not improve the pharmacokinetics of FVIII concentrate in a clinically significant manner
Janneke I. Loomans, Eva Stokhuijzen, Marjolein Peters, Karin Fijnvandraat
Loomans et al., J Clin Transl Res, 2017, 3(S2): 3
Published online: February 21, 2018
Abstract
Background: The half-life and mean residence time (MRT) of infused recombinant factor VIII (FVIII) concentrate are associated with pre-infusion levels of von Willebrand factor (VWF) in severely affected hemophilia A patients. It is currently unknown if individual FVIII concentrate half-life and MRT can be extended by increasing endogenous VWF levels.
Aim: Our aim was to evaluate the effect of a 1-deamino-8-D-arginine vasopressin (DDAVP)-induced rise in VWF concentration on the pharmacokinetics of infused FVIII in hemophilia A patients.
Methods: Four adult hemophilia A patients participated in this cross-over, placebo controlled study. Each patient received either intravenous DDAVP or placebo, one hour prior to administration of 50 IU/kg plasma-derived immune-affinity purified FVIII concentrate.
Results: The combined administration of DDAVP and FVIII concentrate was well tolerated. The levels of VWF Antigen (Ag) doubled after DDAVP, whereas they remained stable after placebo infusion. This rise in VWF Ag resulted in a slight modification of the pharmacokinetic parameters of FVIII concentrate. The MRT of FVIII concentrate increased in all patients (mean from 17.6 h to 19.9 h, p < 0.001, 95% CI for MRT change: +4.7 to -0.3 h). However, in vivo recoveries tended to decrease following DDAVP administration.
Conclusions: Collectively, these data show that administration of DDAVP did not improve the pharmacokinetics of FVIII concentrate in a clinically significant manner.
Relevance for patients: Our results indicate that no clinical benefit is to be expected from the modification in FVIII pharmacokinetics resulting from DDAVP-administration prior to infusion of FVIII concentrate in hemophilia A patients.
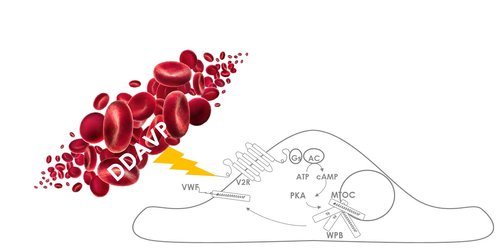
DOI: http://dx.doi.org/10.18053/jctres.03.2017S2.003
Author affiliation
1. Emma Children’s Hospital, Department of Pediatric Hematology, Academic Medical Center, University of Amsterdam, Amsterdam, the Netherlands
2. Department of Plasma Proteins, Sanquin Research, Amsterdam, the Netherlands
*Corresponding author
Janneke Loomans
Emma Children’s Hospital, Department of Pediatric Hematology, Academic Medical Center, University of Amsterdam, Amsterdam, the Netherlands
Email: j.i.loomans@amc.uva.nl
Handling editor:
Michal Heger
Department of Experimental Surgery, Academic Medical Center, University of Amsterdam, Amsterdam, the Netherlands

