-
Home
-
About JCTR
-
Gold Open Access
-
Issues
-
Editorial board
-
Author guidelines
-
Publication fees
-
Online first
-
Special issues
-
News
-
Publication ethics
-
Partners
-
Submit your manuscript
-
Submit your review report
-
Editorial Office
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. ISSN print: 2382-6533 ISSN online: 2424-810X
Volume 3 Special issue on ICT and Healthcare
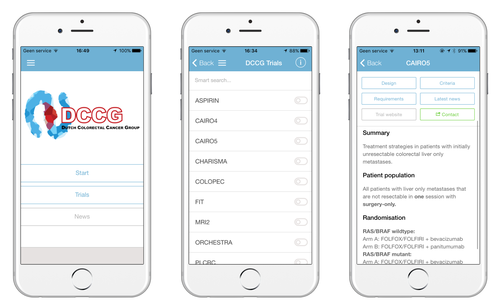
Keeping track of colorectal cancer trials with the Dutch Colorectal Cancer Group mobile app
Joost Huiskens, Michael S. Gałek-Aldridge, Jean-Michel Bakker, Pim B. Olthof, Thomas M. van Gulik, Cornelis J.A Punt, Martijn G.H. van Oijen
Huiskens et al., J Clin Transl Res, 2017; S3: 7
Published online: December 16, 2018
Abstract
Background and Aim: Both the number and complexity of medical trials are increasing vastly. To facilitate easy access to concise trial information, a freely available mobile application featuring all ongoing clinical trials of the Dutch Colorectal Cancer Group (DCCG) was developed. The aim of this study was to investigate the use and user satisfaction over the first two years.
Methods: The application was launched in January 2015 on iOS and Android platforms. Google Analytics was used to monitor anonymous user data up to February 2017. In addition, an online survey regarding the use and satisfaction amongst healthcare professionals and research affiliates active in the field of colorectal cancer in the Netherlands was conducted.
Results: A total of 6,173 unique users were identified, of which 1,822 (30%) were from the Netherlands, representing a total of 16,065 and 10,987 (68%) sessions, respectively. The median session duration per day was 01:47 minutes (IQR 0:51-03:03). The mobile application was most used on Monday, Tuesday, and Thursday and the number of sessions was highest during the following time frames: 12:00-13:00 (9%), 17:00-18:00 (9%), and 13:00-14:00 (8%). Out of 121 survey responses, most were medical doctors (47%), nurses (25%), or researchers (9%); working either in a teaching (40%), academic hospital (32%), or general hospital (19%). Eighty-three percent of all respondents rated the application 4 or higher for satisfaction on a 5-point scale. Highest reported reasons of use were: urgent trial inquiry (57%) and usage during multi-disciplinary meetings (49%).
Conclusion: The DCCG Trials App is frequently used and the majority of users is highly satisfied.
Relevance for patients: Clustering trial information into one platform, such as DCCG Trials App, has shown to be useful for medical professionals treating patients with colorectal carcinoma in the Netherlands.
DOI: http://dx.doi.org/10.18053/jctres.03.2017S3.007
Author affiliation
1. Department of Surgery, Amsterdam University Medical Center, University of Amsterdam, Amsterdam, the Netherlands
2. Department of Medical Oncology, Amsterdam University Medical Center, University of Amsterdam, Amsterdam, the Netherlands
3. Department of Surgery, Reinier de Graaf Gasthuis, Delft, the Netherlands
*Corresponding author:
J. Huiskens
Department of Medical Oncology,
Amsterdam University Medical Center
Department of Medical Oncology
Meibergdreef 9 1105 AZ, Amsterdam
Email: j.huiskens@amc.uva.nl
Tel: +31 (20) 56 63 995
Handling editor:
Michal Heger
Department of Experimental Surgery, Academic Medical Center, University of Amsterdam, Amsterdam, the Netherlands

