-
Home
-
About JCTR
-
Gold Open Access
-
Issues
-
Editorial board
-
Author guidelines
-
Publication fees
-
Online first
-
Special issues
-
News
-
Publication ethics
-
Partners
-
Submit your manuscript
-
Submit your review report
-
Editorial Office
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. ISSN print: 2382-6533 ISSN online: 2424-810X
Volume 4 Issue 1
Mitochondrial dysfunction as a mechanism of drug-induced hepatotoxicity: current understanding and future perspectives
Anup Ramachandran, Luqi Duan, Jephte Y. Akakpo, Hartmut Jaeschke
Ramachandran et al., J Clin Transl Res 2018; 4(1): 5
Published online: May 28, 2018
Abstract
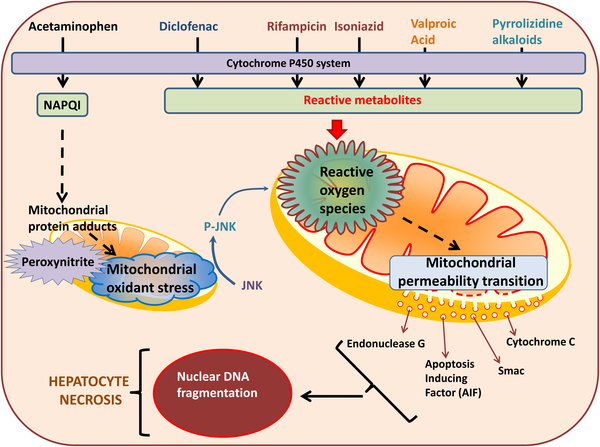
Mitochondria are critical cellular organelles for energy generation and are now also recognized as playing important roles in cellular signaling. Their central role in energy metabolism, as well as their high abundance in hepatocytes, make them important targets for drug-induced hepatotoxicity. This review summarizes the current mechanistic understanding of the role of mitochondria in drug-induced hepatotoxicity caused by acetaminophen, diclofenac, anti-tuberculosis drugs such as rifampin and isoniazid, anti-epileptic drugs such as valproic acid and constituents of herbal supplements such as pyrrolizidine alkaloids. The utilization of circulating mitochondrial-specific biomarkers in understanding mechanisms of toxicity in humans will also be examined. In summary, it is well-established that mitochondria are central to acetaminophen-induced cell death. However, the most promising areas for clinically useful therapeutic interventions after acetaminophen toxicity may involve the promotion of adaptive responses and repair processes including mitophagy and mitochondrial biogenesis, In contrast, the limited understanding of the role of mitochondria in various aspects of hepatotoxicity by most other drugs and herbs requires more detailed mechanistic investigations in both animals and humans. Development of clinically relevant animal models and more translational studies using mechanistic biomarkers are critical for progress in this area.
Relevance for patients: This review focuses on the role of mitochondrial dysfunction in liver injury mechanisms of clinically important drugs like acetaminophen, diclofenac, rifampicin, isoniazid, amiodarone and others. A better understanding of the mechanisms in animal models and their translation to patients will be critical for the identification of new therapeutic targets.
DOI: http://dx.doi.org/10.18053/jctres.04.201801.005
Author affiliation
Department of Pharmacology, Toxicology & Therapeutics, University of Kansas Medical Center, Kansas City, KS, United States
*Corresponding author:
Hartmut Jaeschke
University of Kansas Medical Center, Department of Pharmacology, Toxicology & Therapeutics, 3901 Rainbow Blvd, MS 1018
Kansas City, KS, 66160, United States
Tel. +1 913 588 7969
Email: hjaeschke@kumc.edu
Handling editor:
Michal Heger
Department of Experimental Surgery, Academic Medical Center, University of Amsterdam,Meibergdreef 9, 1105 AZ Amsterdam, the Netherlands

