-
Home
-
About JCTR
-
Gold Open Access
-
Issues
-
Editorial board
-
Author guidelines
-
Publication fees
-
Online first
-
Special issues
-
News
-
Publication ethics
-
Partners
-
Submit your manuscript
-
Submit your review report
-
Editorial Office
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. ISSN print: 2382-6533 ISSN online: 2424-810X
Volume 5 Issue 3
Thermodynamic profiling during irreversible electroporation in porcine liver and pancreas: a case study series
Pierre Agnass, Eran van Veldhuisen, Jantien A. Vogel, H. Petra Kok, Mark J. de Keijzer, Gerben Schooneveldt, Lianne R. de Haan, John H. Klaessens, Hester J. Scheffer, Martijn R. Meijerink, Krijn P. van Lienden, Thomas M. van Gulik, Michal Heger, Johannes Crezee, Marc G. Besselink
Agnass et al., J Clin Transl Res 2020; 5(3): 4
Published online: March 12, 2020
Abstract
Aims: Firstly, to determine whether irreversible electroporation (IRE) is associated with heat generation in the liver and pancreas at clinical (≤ 1,500 V/cm) and supraclinical (> 1,500 V/cm) electroporation settings. Secondly, to assess the risk of thermal tissue damage in and adjacent to the treated volume in highly perfused versus moderately perfused parts of both organs. Thirdly, to investigate the influence of perfusion and of the presence and the orientation of a metal stent on the maximal thermal elevation (ΔTSession,max) in the tissue during an IRE session at fixed IRE settings. Finally, to determine whether the maximum temperature elevation within the IRE-subjected organ during an IRE treatment (single or multiple sessions) is reflected in the organ’s surface temperature.
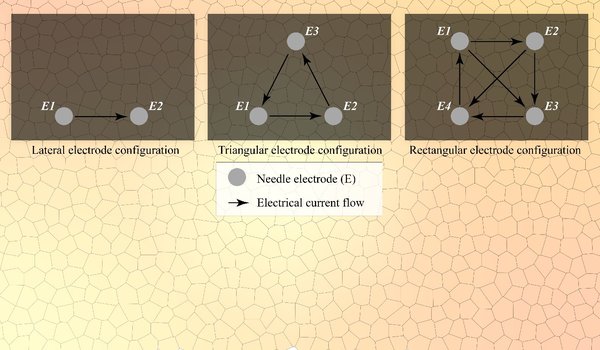
Methods: The aims were investigated in 12 case studies conducted in 5 female Landrace pigs. Several IRE settings were applied for lateral (2), triangular (3), and rectangular (4) electrode configurations in the liver hilum, liver periphery, pancreas head, and pancreas tail. IRE series of 10 - 90 pulses were applied with pulse durations that varied from 70 µs - 90 µs and electric field strengths between 1,200 V/cm - 3,000 V/cm. In select cases, a metal stent was positioned in the bile duct at the level of the liver hilum. Temperatures were measured before, during, and after IRE in and adjacent to the treatment volumes using fiber optical temperature probes (temperature at the nucleation centers) and digital thermography (surface temperature). The occurrence of thermal damage was assumed to be at temperatures above 50 °C (ΔTSession,max ≥ 13 °C relative to body temperature of 37 °C). The temperature fluctuations at the organ surface (ΔTLocSurf) were compared to the maximum temperature elevation during an IRE treatment in the electroporation zone. In select cases, IRE was applied to tissue volumes encompassing the portal vein (PV) and a constricted and patent superior mesenteric vein (SMV) to determine the influence of the heatsink effect of PV and SMV on ΔTSession,max.
Results: The median baseline temperature was 31.6 °C - 36.3 °C. ΔTSession,max ranged from –1.7 °C - 25.5 °C in moderately perfused parts of the liver and pancreas, and from 0.0 °C - 5.8 °C in highly perfused parts. The median ΔTLocSurf of the liver and pancreas was 1.0 °C and 10.3 °C, respectively. Constricting the SMV in the pancreas head yielded a 0.8 °C higher ΔTSession,max. The presence of metal stents in the liver hilum resulted in a ΔTSession,max of 19.8 °C. Stents parallel to the electrodes caused a ΔTSession,max difference of 4.2 °C relative to the perpendicular orientation.
Conclusions: Depending on IRE settings and tissue type, IRE is capable of inducing considerable heating in the liver and pancreas that is sufficient to cause thermal tissue damage. More significant temperature elevations are positively correlated with increasing number of electrode pairs, electric field strength, and pulse number. Temperature elevations can be further exacerbated by the presence and orientation of metal stents. Temperature elevations at the nucleation centers are not always reflected in the organ’s surface temperature. Heat sink effects caused by large vessels were minimal in some instances, possibly due to reduced blood flow caused by anesthesia.
Relevance for patients: Appropriate IRE settings must be chosen based on the tissue type and the presence of stents to avoid thermal damage in healthy peritumoral tissue and to protect anatomical structures.
DOI:http://dx.doi.org/10.18053/jctres.05.202003.004
Author affiliation
1 Department of Surgery, Cancer Center Amsterdam, Amsterdam UMC, University of Amsterdam, Amsterdam, The Netherlands
2 Department of Radiation Oncology, Cancer Center Amsterdam, Amsterdam UMC, University of Amsterdam, Amsterdam, The Netherlands
3 Department of Experimental Surgery, Amsterdam UMC, University of Amsterdam, Amsterdam, The Netherlands
4 Department of Pharmaceutics, Utrecht Institute for Pharmaceutical Sciences, Utrecht University, Utrecht, The Netherlands
5 Department of Clinical Physics, Medical Center Leeuwarden, Leeuwarden, The Netherlands
6 Department of Radiology and Nuclear Medicine, Cancer Center Amsterdam, Amsterdam UMC, Vrije Universiteit Amsterdam, Amsterdam, The Netherlands
7 Department of Radiology, Cancer Center Amsterdam, Amsterdam UMC, University of Amsterdam, Amsterdam, The Netherlands
8 Department of Pharmaceutics, College of Medicine, Jiaxing University, Jiaxing, Zhejiang, P.R. China
* Corresponding author:
M.G. Besselink
Cancer Center Amsterdam, Amsterdam UMC, University of Amsterdam, Department of Surgery Meibergdreef 9, 1100 DD Amsterdam, The Netherlands
Tel: +31 20 56 22571
E-mail: m.g.besselink@amsterdamumc.nl
Correspondence during review: p.agnass@amsterdamumc.nl
Handling editor:
Rowan van Golen
Gastroenterology and Hepatology, Leiden University Medical Center, the Netherlands

