-
Home
-
About JCTR
-
Gold Open Access
-
Issues
-
Editorial board
-
Author guidelines
-
Publication fees
-
Online first
-
Special issues
-
News
-
Publication ethics
-
Partners
-
Submit your manuscript
-
Submit your review report
-
Editorial Office
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. ISSN print: 2382-6533 ISSN online: 2424-810X
Volume 7 Issue 1
Interleukin-13 as a target to alleviate severe coronavirus disease 2019 and restore lung homeostasis
Lachlan Paul Deimel*, Zheyi Li and Charani Ranasinghe
Deimel et al. J Clin Transl Res 2020; 7(1):4
Published online: January 27, 2021
Abstract
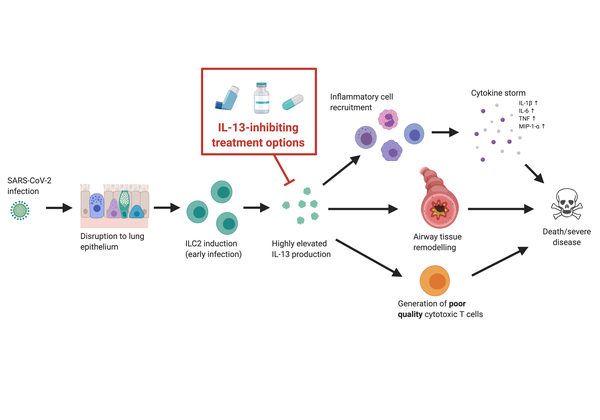
The ongoing COVID-19 pandemic urgently requires the availability of interventions that improve outcomes for those with severe disease. Since SARS-CoV-2 infection is characterised by dysregulated lung mucosae, and that mucosal homeostasis is heavily influenced by IL-13 activity, we explore recent findings indicating that IL-13 production is proportional to disease severity. We propose that excessive IL-13 contributes to the progression of severe/fatal COVID-19 by 1) promoting the recruitment of immune cells that express inflammatory cytokines, causing a cytokine storm that results in widespread destruction of lung tissue, 2) directly facilitating tissue-remodelling that causes airway hyperinflammation and obstruction, and 3) diverting the immune system away from developing high-quality cytotoxic T cells that confer effective anti-viral immunity. These factors may cumulatively result in significant lung distress, multi-organ failure and death. Here, we suggest repurposing existing IL-13-inhibiting interventions, including antibody therapies routinely used for allergic lung hyperinflammation, as well as viral vector-based approaches, to alleviate disease. Since many of these strategies have previously been shown to be both safe and effective, this could prove to be a highly cost-effective solution.
Relevance for patients: There remains a desperate need to establish medical interventions that reliably improves outcomes for patients suffering from COVID-19. We explore the role of IL-13 in maintaining homeostasis at the lung mucosae and propose that its dysregulation during viral infection may propagate the hallmarks of severe disease––further exploration may provide a platform for invaluable therapeutics.
DOI: http://dx.doi.org/10.18053/jctres.07.202101.004
Author affiliation
Molecular Mucosal Vaccine Immunology Group, Department of Immunology and Infectious Disease, The John Curtin School of Medical Research, The Australian National University, Canberra ACT 2601, Australia
*Corresponding author
Lachlan Paul Deimel
Molecular Mucosal Vaccine Immunology Group, Department of Immunology and Infectious Disease, The John Curtin School of Medical Research, The Australian National University, Canberra ACT 2601, Australia
Tel: +61 2 6125 4706
Email: Lachlan.Deimel@anu.edu.au
Handling editor:
Michal Heger
Department of Pharmaceutics, Utrecht University, the Netherlands
Department of Pharmaceutics, Jiaxing University Medical College, Zhejiang, China

