-
Home
-
About JCTR
-
Gold Open Access
-
Issues
-
Editorial board
-
Author guidelines
-
Publication fees
-
Online first
-
Special issues
-
News
-
Publication ethics
-
Partners
-
Submit your manuscript
-
Submit your review report
-
Editorial Office
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. ISSN print: 2382-6533 ISSN online: 2424-810X
Volume 7 Issue 1
Can tetracyclines ensure help in multiple sclerosis immunotherapy?
Pedro Víctor-Carvalho, Rodolfo Thome, Catarina Rapôso*
Victor-Carvalho et al. J Clin Transl Res. 2021; 7(1):10
Published online: February 3, 2021
Abstract
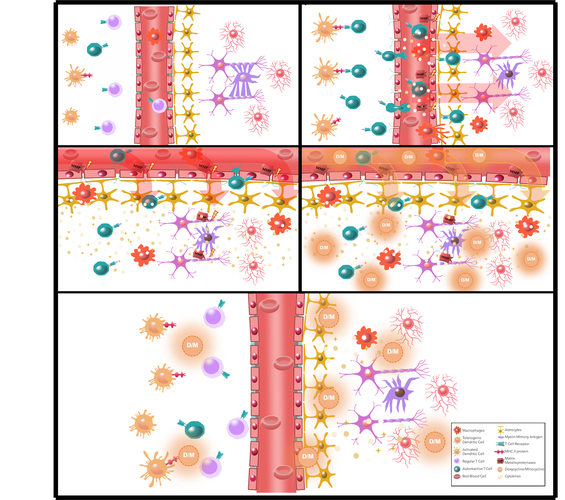
Background. Multiple Sclerosis (MS) is a disease of the central nervous system where an autoimmune response leads to chronic inflammation. It represents the second leading cause of non-traumatic disability in the world, affecting mainly young adults and with high female to male incidence. Currently, the causative agent in MS is unknown, preventing the development of prophylaxis policies and the understanding of how the human system copes with this complex inflammation. Tetracyclines have attracted great attention due to their anti-inflammatory effects. Minocycline and Doxycycline represent the second-generation tetracyclines that have been largely used to treat acne and to suppress inflammation. Additionally, they are safer and cheaper than other drugs currently used to treat MS.
Aim. This study aims to review recent data involving the tetracyclines Minocycline and Doxycycline and their therapeutic potential in MS.
Relevance for patients. Many of the drugs used to treat MS have severe side effects and are costly. Tetracyclines, on the other hand, are a safe and inexpensive class of drugs that can modulate the immune response in MS patients.
Keywords: Doxycycline, Minocycline, immunomodulation, nonantibiotic actions, neuroprotection.
DOI: http://dx.doi.org/10.18053/jctres.07.202101.010
Author affiliation
1. Department of Structural and Functional; BiologyInstitute of Biology, University of Campinas (UNICAMP), Campinas, SP, Brazil.
2. Department of Neurology, Thomas Jefferson University, Philadelphia-PA, USA.
3. Laboratory of Drug Development, Faculty of Pharmaceutical Sciences, University of Campinas (UNICAMP), Campinas, SP, Brazil.
*Corresponding author
Catarina Rapôso
Faculty of Pharmaceutical Sciences, University of Campinas, (UNICAMP), 13083-87, Campinas, SP, Brazil.
Email: raposo@unicamp.br
Handling editor:
Michal Heger
Department of Pharmaceutics, Utrecht University, the Netherlands
Department of Pharmaceutics, Jiaxing University Medical College, Zhejiang, China

