-
Home
-
About JCTR
-
Gold Open Access
-
Issues
-
Editorial board
-
Author guidelines
-
Publication fees
-
Online first
-
Special issues
-
News
-
Publication ethics
-
Partners
-
Submit your manuscript
-
Submit your review report
-
Editorial Office
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. ISSN print: 2382-6533 ISSN online: 2424-810X
Volume 7 issue 2
Addressing the global surge of COVID-19 cases: insights from diagnostics, improved treatment strategies, vaccine development and application
Kamoru A. Adedokun, Ayodeji O. Olarinmoye*, Lawal O. Olayemi*, Muhammed R. Shehu , Jelili O. Mustapha, Ramat T. Kamorudeen, Sulaimon A. Nassar
Adedokun et al. J Clin Transl Res 2021; 7(2):2
Published online: 12 March, 2021
Abstract
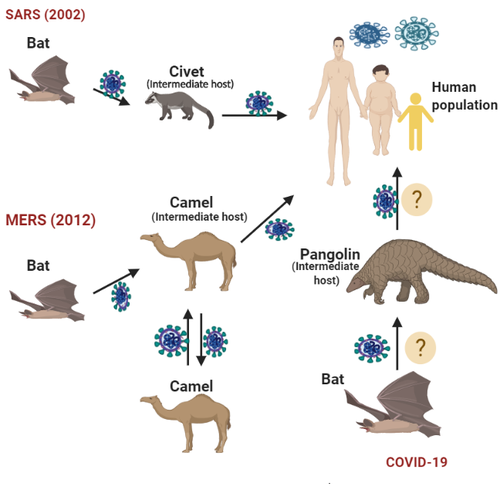
Background and aim: As the rage of coronavirus disease 2019 (COVID-19) pandemic continues to spread globally much effort is being directed to contain it through various efforts - genomic studies, drug discoveries, clinical trials, vaccine development, and the innovation of diagnostic techniques. However, some pertinent areas involving accurate and sensitive diagnostics, immunoglobulin specificity, evolution of mutant strains of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and the drug combination strategy to combat it still require more attention.
Methods: This review critically examines the COVID-19 response and containment operations. It also addresses some standing challenges involving the areas of diagnostics, vaccine development and prospect, and treatment strategies in relation to antiviral drug treatment and immunotherapy. Designated set of keywords such as ‘SARS-CoV-2’; ‘coronavirus’; ‘severe acute respiratory syndrome coronavirus’; ‘repurposed’; ‘vaccination’; ‘containment’; ‘laboratory diagnostic’; ‘immunotherapy’; ‘antiviral’; ‘antiparasitic’; ‘antibiotic’; ‘antiprotozoal’; ‘antibody’; ‘anti-inflammatory’; ‘antitumour’; ‘corticosteroid’; ‘hypertensive drug’; ‘statin’; ‘supplement’; ‘biological’ along with ‘COVID-19’ were inserted on electronic databases to retrieve articles and clinical trial information relevant to the study objectives. The search databases include ClinicalTrials.gov, NIH.gov, PubMed, Scinapse, CINAHL, Medline, Google Scholar, Academic Search Premier, SAGE, EBSCO Host, and Scopus.
Relevance for patients: The difficulties associated with SARS-CoV-2 rapid mutations are unceasingly evolving and re-evolving. These pose serious concerns and downplay the efficacy and effectiveness of the current pipeline antiviral drugs and vaccines. Entities encompassing immunotherapy, antiviral drug therapies, viral genomics, protein-protein interaction and improved diagnostics as well as drug combination strategy against the emerging genetic variability of SARS-CoV-2 were critically appraised. This study suggests that robust collaborations in the development of more sensitive, rapid and accurate diagnostics, development of immunoglobulin specific agents and improved anti-viral treatment focus against multiple mutant genes of SARS-COV-2 should be aggressively pursued for the overall benefits of the patients.
DOI: http://dx.doi.org/10.18053/jctres.07.202102.002
Author affiliation
1. Department of Oral Pathology, DUH, King Saud University Medical City, Riyadh, Saudi Arabia,
2. Department of Agriculture and Industrial Technology, Babcock
University, Ilisan Remo, Ogun State, Nigeria,
3. School of Medicine, Faculty of Health Sciences, National University of Samoa, Samoa,
4. Department of Environmental
Science, Southern Illinois University, Edwardsville, Illinois USA,
5. Molecular Diagnostics Unit, DynaLIFE Medical Labs, Edmonton, Alberta, Canada, 6
Department
of Public Health, University of South Wales, Pontypridd, UK,
7. Children Welfare Unit, Osun State Hospital Management Board, Asubiaro, Osogbo, Osun State,
Nigeria,
8. Department of Medical Laboratory Sciences, College of Health Sciences, Ladoke Akintola University, Ogbomoso, Oyo State, Nigeria.
* Corresponding Authors
Lawal O. Olayemi
School of Medicine, Faculty of Health Sciences, National University of Samoa,
P.O.Box 1622, Lepapaigalagala, To’omatagi, Apia, Samoa, South Pacific
Tel: +685 7513234Email: olayemis2002@yahoo.com
Ayodeji O. Olarinmoye
Department of Agriculture and Industrial Technology, School of Science and Technology, Babcock University, Ogun State, Nigeria.
Tel: +2348063510601
Email: aolarinmoye@yahoo.com
Handling editor:
Michal Heger
Department of Pharmaceutics, Utrecht University, the Netherlands
Department of Pharmaceutics, Jiaxing University Medical College, Zhejiang, China

