-
Home
-
About JCTR
-
Gold Open Access
-
Issues
-
Editorial board
-
Author guidelines
-
Publication fees
-
Online first
-
Special issues
-
News
-
Publication ethics
-
Partners
-
Submit your manuscript
-
Submit your review report
-
Editorial Office
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. ISSN print: 2382-6533 ISSN online: 2424-810X
Volume 9 Issue 6
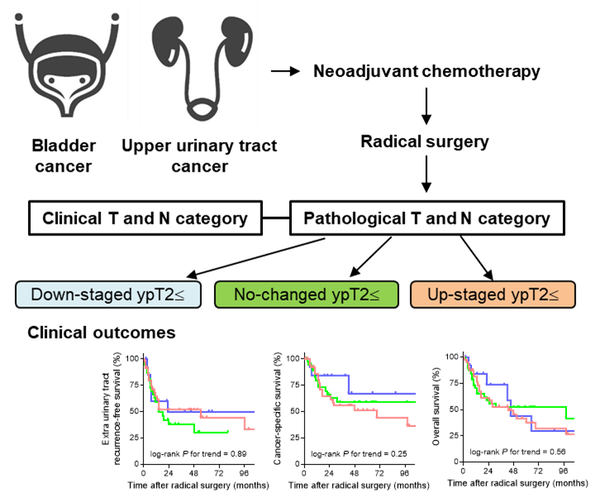
Association between response to neoadjuvant chemotherapy and survival outcome after radical surgery in patients with yielding pathological T2≤ and/or N+ urothelial carcinoma
Makito Miyake*, Nobutaka Nishimura, Nagaaki Marugami, Tomomi Fujii, Yuya Fujiwara, Kazumasa Komura, Teruo Inamoto, Haruhito Azuma, Hiroaki Matsumoto, Hideyasu Matsuyama, Kiyohide Fujimoto, On behalf of Nishinihon Uro-Oncology Collaborative Group
Miyake et al. J Clin Transl Res 2023; 9(6):23-00106
Published online: November 12, 2023
Abstract
Background and Aim: In early 2022, the use of adjuvant nivolumab for patients with high-risk muscle-invasive urothelial carcinoma was approved in Japan, European countries, and USA based on the positive results of CheckMate 274 trial, which included participants who received neoadjuvant chemotherapy (NAC). Subgroup analyses of CheckMate 274 trial does not report response to neoadjuvant chemotherapy and benefit from adjuvant nivolumab. Herein, we investigated the association between response to NAC and survival outcomes after radical surgery in patients with residual muscle-invasive urothelial carcinoma and/or lymph node disease.
Methods: This multicenter retrospective study included a total of 95 NAC-treated patients with yielding pathological (yp) T2≤ and/or ypN+ urothelial carcinoma on radical surgery specimens. Based on the comparison of clinical T and N category with yp T and N category, the patients were categorized into three groups: down-staged ypT2≤ (n=14), no-changed ypT2≤ (n=39), and up-staged ypT2≤ groups (n=42).
Results: There was no significant difference in extra-urinary tract recurrence-free survival, cancer-specific survival, and overall survival after the radical surgery among three groups. Subgroup analysis of a bladder cancer cohort showed a marginal association between better response and longer cancer-specific survival (P=0.073).
Conclusion: Our finding suggested that adjuvant nivolumab should be considered for all the patients with pathological ypT2≤ or ypN+ urothelial carcinoma regardless of response to NAC. Further research is mandatory in finding predictive factors that serve in decision-making for NAC-treated patients who are likely to benefit from adjuvant nivolumab.
Relevance for patients: To develop a decision-making tool for adjuvant nivolumab, we investigated the association between response to neoadjuvant chemotherapy and survival after radical surgery. Further research is mandatory in finding predictive factors that serve in decision-making for NAC-treated patients who are likely to benefit from adjuvant nivolumab.
DOI: http://dx.doi.org/10.18053/jctres.09.202306.23-00106
Author affiliation
1. Department of Urology, Nara Medical University, Kashihara, Nara, 634-8522, Japan
2. Department of Radiology, Nara Medical University, Kashihara, Nara, Japan
3. Department of Diagnostic Pathology, Nara Medical University, Kashihara, Nara, 634-8522, Japan
4. Department of Urology, Osaka Medical and Pharmaceutical University, Takatsuki, Japan
5. Department of Urology, Graduate School of Medicine, Yamaguchi University, Ube, Yamaguchi, Japan
6. Department of Urology, JA Yamaguchi Kouseiren Nagato General Hospital, Yamaguchi, Japan
*Corresponding author
Makito Miyake
Department of Urology, Nara Medical University, 840 Shijo-cho, Nara 634-8522, Japan.
Tel: +81-744-22-3051 (ext 2338)
Fax: +81-744-22-9282
E-mail: makitomiyake@yahoo.co.jp
Handling editor:
Michal Heger
Department of Pharmaceutics, Utrecht University, the Netherlands
Department of Chemistry, Utrecht University, Utrecht, the Netherlands
Department of Pathology, Erasmus Medical Center, the Netherlands
Department of Pharmaceutics, Jiaxing University Medical College, Zhejiang, China

