-
Home
-
About JCTR
-
Gold Open Access
-
Issues
-
Editorial board
-
Author guidelines
-
Publication fees
-
Online first
-
Special issues
-
News
-
Publication ethics
-
Partners
-
Submit your manuscript
-
Submit your review report
-
Editorial Office
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. ISSN print: 2382-6533 ISSN online: 2424-810X
Volume 1 Issue 2
TEMPOL has limited protective effects on renal oxygenation and hemodynamics but reduces kidney damage and inflammation in a rat model of renal ischemia/reperfusion by aortic clamping
Bulent Ergin, Rick Bezemer, Asli Kandil, Cihan Demirci-Tansel, Can Ince
Ergin et al., J Clin Transl Res, 2015; 1(2): 116-128
Published online: 29 September, 2015
Corrigendum:
Page 125, Acknowledgment: "This research was partially supported by Scientific Research
Projects Coordination Unit of Istanbul University (Project
number: UDP-25344)." should read: "This research was partially supported by Scientific Research
Projects Coordination Unit of Istanbul University (Project
number: UDP-25511)."
Abstract
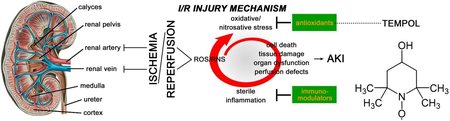
Background: Renal ischemia-reperfusion (I/R) is a common clinical complication in critically ill patients that is associated with considerable morbidity and mortality. Renal I/R is a major cause of acute kidney injury (AKI) resulting from I/R-induced oxidative stress, sterile inflammation, and microcirculatory perfusion defects, which can be ameliorated with the superoxide scavenger TEMPOL. The most common cause of AKI in the clinical setting is aortic surgery with suprarenal aortic clamping. The protective effect of TEMPOL in aortic clamping-induced renal I/R has not been studied before.
Aim: To evaluate the protective effects of TEMPOL on oxidative stress, inflammation, tissue injury, and renal hemodynamics and oxygenation in a clinically representative rat model of I/R using aortic cross-clamping.
Methods: Animals (N = 24) were either sham-operated or subjected to ischemia (30 min) and 90-min reperfusion, with or without TEMPOL treatment (15 min before ischemia and during entire reperfusion phase, 200 μmol/kg/h). Systemic and renal hemodynamics, renal oxygenation, and blood gas values were determined at 15min and 90min of reperfusion. At 90-min reperfusion, iNOS, inflammation (IL-6, MPO), oxidative stress (MPO), and tissue damage (NGAL, L-FABP) were determined in tissue biopsies.
Results: TEMPOL administration at a cumulative dose of 400 μmol/kg conferred a protective effect on AKI in terms of reducing renal damage, inflammation, and iNOS activation. With respect to renal hemodynam-ics and oxygenation, TEMPOL only reduced renal vascular resistance to near-baseline levels at both reperfusion time points and partially ameliorated the I/R-induced drop microvascular partial tension of oxygen at 90 min reperfusion. Also, TEMPOL alleviated the I/R-induced metabolic acidosis. However, TEMPOL exerted no restorative effect in terms of the severely reduced mean arterial pressure, renal blood flow, and renal oxygen delivery and consumption. The renal oxygen extraction ratio remained unchanged during the 90-min reperfusion phase. Kidneys in all groups were anuric throughout the experiment.
Conclusions: This clinically representative renal I/R model, which entails both renal I/R and hind limb I/R as opposed to the standardly used renal I/R model that employs renal artery clamping, resulted in relatively moderate direct AKI. The damage was exacerbated by the perturbed systemic hemodynamics and metabolic acidosis as a result of the hind limb I/R. TEMPOL partially intervened in the factors that lead to AKI as well as renal microvascular partial tension of oxygen and metabolic acidosis. However, more effective interventions should be devised for the mean arterial pressure drop (i.e., anuria) associated with aortic clamping and for restoring other critical renal hemodynamic and oxygenation parameters in order to improve post-I/R renal function.
Relevance for patients: TEMPOL is a promising compound that has been shown to protect kidneys from I/R damage, which is relevant in kidney transplantation, pancreas transplantation, and aortic aneurysm repair in kidney transplant patients. This study suggests that intervening with TEMPOL is not sufficient to ensure optimal clinical outcome in patients that have undergone aortic clamping and that more effective interventions should be investigated.
DOI: http://dx.doi.org/10.18053/jctres.201502.002
Author affiliation
1 Department of Translational Physiology, Academic Medical Center, University of Amsterdam, Amsterdam, The Netherlands
2 Department of Biology, Faculty of Science, Istanbul University, Vezneciler, Istanbul, Turkey
Corresponding author:
Bulent Ergin
Department of Translational Physiology, Academic Medical Center, University of Amsterdam, Meibergdreef 9, 1105 AZ Amsterdam, The Netherlands
Tel: +31-651077925
Email: b.ergin@amc.uva.nl

