-
Home
-
About JCTR
-
Gold Open Access
-
Issues
-
Editorial board
-
Author guidelines
-
Publication fees
-
Online first
-
Special issues
-
News
-
Publication ethics
-
Partners
-
Submit your manuscript
-
Submit your review report
-
Editorial Office
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. ISSN print: 2382-6533 ISSN online: 2424-810X
Volume 3 Special Issue 1
Mechanisms of Drug-induced Hepatotoxicity: A Translational Research Perspective
Editors
Prof. Hartmut Jaeschke
Dr. Anup Ramachandran
University of Kansas Medical Center, United States
Published now!
Preface
Hartmut Jaeschke, Anup Ramachandran
Drug hepatotoxicity is a significant problem during drug development and in clinical practice. Fundamentally, drug-induced liver injury can be predictable and dose-dependent, e.g. acetaminophen hepatotoxicity; or rare and unpredictable (idiosyncratic), e.g. troglitazone. In either case, understanding mechanisms of toxicity, identifying therapeutic targets and developing biomarkers that allow early detection in animals and humans is vital to limit this problem. The current issue of the Journal of Clinical and Translational Research provides a number of reviews that summarize the current state of knowledge in this field with an emphasis on the translation to human conditions. In the first article, Mak and Uetrecht [1] describe our understanding of drug-induced idiosyncratic hepatotoxicity. This is the most challenging area for preclinical safety assessment as well as clinical management as a solid mechanistic understanding of this toxicity is lacking largely due to absence of reliable animal models. In contrast, acetaminophen-induced hepatotoxicity and acute liver failure is the most frequent cause of drug-induced liver injury in the western world and is well studied in animal models for several decades. Two reviews cover the intracellular signaling events caused by acetaminophen overdose leading to liver injury [2] and the resulting sterile inflammatory response after necrotic cell death [3]. Maes et al. [4] discuss the emerging evidence for the roles of connexin-based channels in drug hepatotoxicity and Clarke et al. [5] review the latest information on biomarkers for predicting drug-induced liver injury and evaluating clinical outcome. Finally, given the growing obesity epidemic worldwide, Massart and co-workers summarize the growing literature on the impact of steatosis on drug hepatotoxicity [6].
It is anticipated that this collection of reviews would provide comprehensive information on the multi-faceted, but clinically relevant issue of drug hepatotoxicity.
References
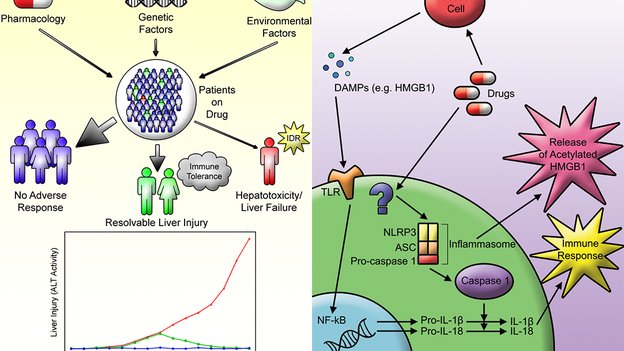
1. Mak A, Uetrecht J. Immune mechanisms of idiosyncratic drug-induced liver injury. J Clin Transl Res; 3(S1): 145-156.
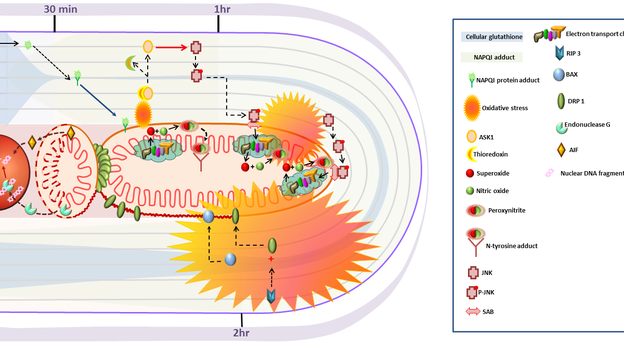
2. Ramachandran A, Jaeschke H. Mechanisms of acetaminophen hepatotoxicity and their translation to the human pathophysiology. J Clin Transl Res; 3(S1): 157-169.
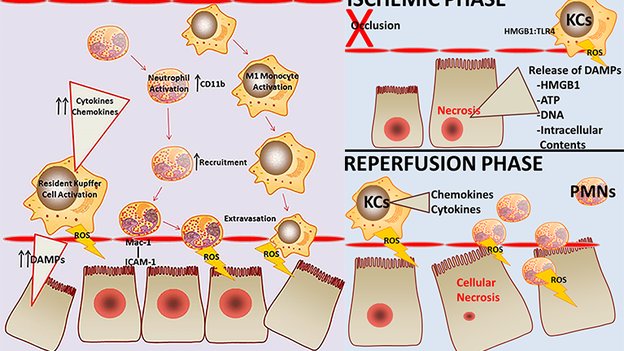
3. Woolbright BL, Jaeschke H. The impact of sterile inflammation in acute liver injury. J Clin Transl Res; 3(S1): 170-188.
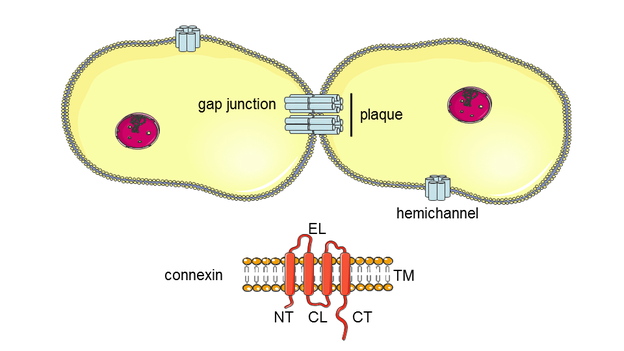
4. Maes M., Vinken M. Connexin-based signaling and drug-induced hepatotoxicity. J Clin Transl Res; 3(S1): 189-198.
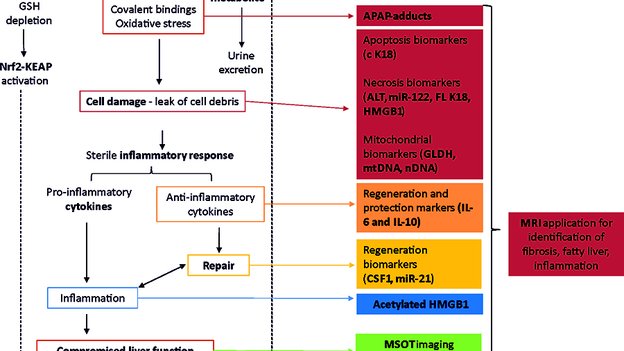
5. Clarke JI, Brillant N, Antoine DJ. Novel circulating- and imaging-based biomarkers to enhance the mechanism understanding of human drug-induced liver injury. J Clin Transl Res; 3(S1): 199-211.
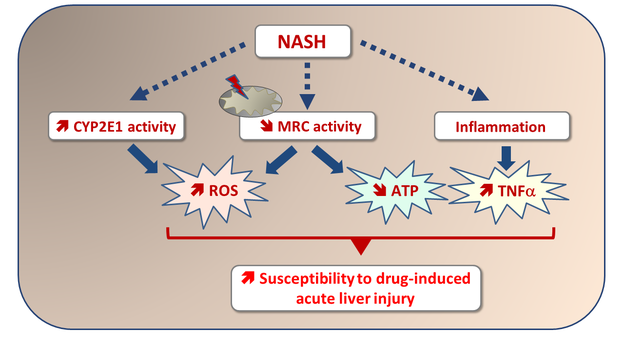
6. Massart J, Begriche K, Moreau C, Fromenty B. Role of nonalcoholic fatty liver disease as risk factor for drug-induced hepatotoxicity. J Clin Transl Res; 3(S1): 212-232.
Biographies of the editors
Hartmut Jaeschke is Professor and Chairman of the Department of Pharmacology, Toxicology, and Therapeutics at the University of Kansas Medical Center, Kansas City, Kansas, USA. He joined KUMC in 2006. He is a Fellow of the Academy of Toxicological Sciences (ATS) and Fellow of the American Association for the Study of Liver Diseases (FAASLD). After receiving his Ph.D. degree in toxicology from the University of Tübingen, Germany, in 1983, he did postdoctoral work at the University of Tübingen and at Baylor College of Medicine in Houston, Texas, USA. Since 1987, he held faculty positions at Baylor (1987–1992), at the University of Arkansas for Medical Sciences, Little Rock, (1999–2002), and at the University of Arizona, Tucson (2002–2006). In addition, he spent 6 years in the pharmaceutical industry (The Upjohn Co. and Pharmacia & Upjohn Co., Kalamazoo, MI), from 1992 to 1998. He has published more than 370 peer-reviewed manuscripts, invited reviews, and book chapters in the areas of liver toxicology and liver pathophysiology. In the past, Dr. Jaeschke served on the Editorial Boards of American Journal of Physiology: Liver and Gastrointestinal Physiology, Gastroenterology, and Toxicology Letters and was an Associate Editor for Hepatology (2002–2006) and Liver International (2007–2012). He currently serves as Associate Editor for Toxicological Sciences (since 2014), the Journal of Clinical and Translational Research (since 2016) and 4 other journals; in addition, he is member of the editorial boards of Hepatology, Journal of Hepatology, American Journal of Pathology, and 14 other journals. He is a Member of many national and international scientific societies including the Society of Toxicology and the American Association for the Study of Liver Diseases. His major research interests include basic mechanisms and translational aspects of xenobiotic-induced hepatotoxicity and tissue repair with focus on acetaminophen overdose, mechanisms of inflammatory liver injury in models of hepatic ischemia-reperfusion injury, obstructive cholestasis, and endotoxin shock, and signaling pathways of apoptosis and programmed necrosis in hepatocytes.
Anup Ramachandran completed his MSc in Medical Biochemistry from the Kasturba Medical College, Mangalore and his Ph.D. from the Christian Medical College, Vellore, India, examining the role of oxygen free radicals and mitochondria in modulating intestinal alterations during surgical stress and liver cirrhosis. He subsequently conducted post-doctoral research at the University of Alabama at Birmingham, in Victor Darley-Usmar’s laboratory, where he delved into the role of the mitochondria in cell signaling, especially in relation to nitric oxide in endothelial cells. He then joined as a Senior Lecturer at the Wellcome Trust Research Laboratory, Christian Medical College, Vellore, where he became Associate Professor in 2009 and Professor of Biochemistry in 2012, while continuing his studies on examining mitochondrial function in a number of pathophysiological conditions including liver cirrhosis, acute fatty liver of pregnancy and organophosphate poisoning. During that period, he spent 3 years working in Dr. Hartmut Jaeschke’s laboratory at the Kansas University Medical Center studying the role of mitochondria in acetaminophen induced hepatotoxicity. He is currently on the Research Faculty at the Kansas University Medical Center, examining the influence of mitochondrial dynamics and signaling on necrotic cell death pathways after acetaminophen overdose.
Review
Immune mechanisms of idiosyncratic drug-induced liver injury
Hepatology
Toxicology
Review
Mechanisms of acetaminophen hepatotoxicity and their translation to the human pathophysiology
Hepatology
Toxicology
Review
The impact of sterile inflammation in acute liver injury
Hepatology
Toxicology
Review
Connexin-based signaling and drug-induced hepatotoxicity
Hepatology
Toxicology
Review
Novel circulating- and imaging-based biomarkers to enhance the mechanistic understanding of human drug-induced liver injury
Hepatology
Toxicology
Review
Role of nonalcoholic fatty liver disease as risk factor for drug-induced hepatotoxicity
Hepatology
Toxicology